Abstract
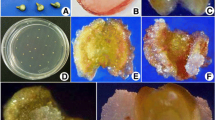
Six experiments (including pretreatment, embryonic callus induction media, preculture conditions, embryo induction media, embryo germination media, and genotypic effects) were conducted to develop an efficient cucumber (Cucumis sativus L., 2n = 2x = 14) anther culture protocol. Pretreatment and embryo induction were key factors for successful anther culture. Suitable temperature stress depended on the ecotype, i.e., cucumbers from cold areas responded well to cold shock whereas those from temperate areas responded well to heat treatment. The best medium for embryonic callus induction was MS medium supplemented with 4.44 μM BA, 2.26 μM 2, 4-D, 4.64 μM KIN, 3% sucrose and 0.8% agar. For embryo induction, MS medium supplemented with 0.54 μM NAA, 13.32 μM BA, 3% sucrose and 0.8% agar was optimal, and for embryo germination MS medium containing 2.22 μM BA, 6% sucrose and 1.2% agar was best. Using this protocol, we produced callus from 16 genotypes and regenerated plants from three of 20 evaluated. Three embryos per anther and 42 DH per 45 anthers (93% success) were obtained for cv. Ningjia No. 1, which was an improved result over a previous report. The origin of regenerants from microspores was determined by cytological, morphological and AFLP analyses.







Similar content being viewed by others
Abbreviations
- R0 :
-
Anther culture derived plants
- R1 :
-
Selfed progeny of R0
- DH:
-
Doubled haploid
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- BA:
-
6-benzyladenine
- KIN:
-
Kinetin
- TDZ:
-
Thidiazuron
- NAA:
-
α-naphthaleneacetic acid
- MS:
-
Murashige and Skoog (1962)
- PGR:
-
Plant growth regulator
References
Amaury M, Arzate-Fernández, Tetsuya N, Hirotada Y, Takatoshi T (1997) Production of doubled-haploid plants from Lilium longiflorum Thunb. anther culture. Plant Sci 123:179–187
Chen JF, Qian CT (2002) Studies on chromosome preparations using plant tendril as source tissue. Acta Hort Sinica 29:378–380
Ernst S, Scheibner K, Diettrich B, Luckner M (1990) Androgenetic cell cultures and plants from anthers of Digitalis lanata. J Plant Physiol 137:129–134
Kumar HGA, Ravishankar BV, Murthy HN (2004) The influence of polyamines on androgenesis of Cucumis sativus L. Eur J Hort Sci 5:201–205
Kumar HGA, Murthy HN (2004) Effect of sugars and amino acids on androgenesis of Cucumis sativus. Plant Cell Tiss Org Cult 78:201–208
Kumar HGA, Murthy HN, Paek KY (2003) Embryogenesis and plant regeneration from anther cultures of Cucumis sativus L. Sci Hort 98:213–222
Lazarte JE, Sasser CC (1982) Asexual embryogenesis plantlet development in anther culture of Cucumis sativus L. HortScience 17:88
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murray HG, Thompson WF (1980) Rapid isolation of higher weight DNA. Nucleic Acids Res 8:4321
Panaud O, Chen X, McCouch SD (1996) Development of microsatellite markers and characterization of simple sequence length polymorphism (SSLP) in rice (Oryza sativa L.). Mol Gen Genet 252:597–607
Rao PS, Suprassanna P (1996) Methods to double haploid chromosome numbers. In: Jain SM, Sopory SK, Veilleux RE (eds) In vitro haploid production in higher plants, vol 1. Kluwer Academic Publ, Dordrecht pp 317–339
SAS Institute Inc. (1999) SAS/STAT user’s guide, version 7-1. SAS Institute Inc, Cary, North Carolina
Vos P, Hogers R, Bleeker M, Reijans M, Van de lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Zhang X, Fowler SG, Cheng HM, Lou YG, Rhee SY, Stockinger EJ, Thomashow MF (2004) Freezing-sensitive tomato has a functional CBF cold response pathway, but a CBF regulon that differs from that of freezing-tolerant Arabidopsis. Plant J 39:905–919
Acknowledgements
This research was supported by the General Program 30470120 and 30671419 from the National Science Foundation of China; the 863 Program 2006AA10Z108, 2006BAD01A7-5-11, 2006AA100108 from the Ministry of Science and Technology of China. We are grateful to Dr Yong-Bing Zhang for his valuable suggestion of the manuscript. I wish to thank the Ms. Hong Geng and Yun-Feng Tan for providing intellectual support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, H., Lou, QF., Luo, XD. et al. Regeneration of doubled haploid plants by androgenesis of cucumber (Cucumis sativus L.). Plant Cell Tiss Organ Cult 90, 245–254 (2007). https://doi.org/10.1007/s11240-007-9263-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-007-9263-y