Abstract
Photocatalysis is a green approach that has appeared to be a viable option for the degradation of a variety of organic contaminants. This work outlines the process of preparing the titanium-based metal-organic framework (MIL-125) photocatalysts using a simple solvothermal method. Structural, morphological, and optical analysis of samples (MT18 and MT48) was carried out by XRD, FT-IR, Raman, SEM, TGA, BET, and UV–Vis. Results indicated that the sample prepared at 150 °C and reaction time of 48 h (MT48) has a low crystal size of 7 nm with an optical band gap of 3.2 eV and a surface area of 301 m2 g−1. Under UV–visible light irradiation, the as-prepared MOFs proved to upgrade photocatalytic activity in degrading crude oil spills in saltwater. Effects of catalyst dosage and exposure time on the degradation of an oil spill in seawater were studied and analyzed using UV–Vis spectrophotometry and gas chromatography (GC–MS) which emphasized that the use of 250 ppm of MT48 photocatalyst under UV–Vis irradiation can degrade about 99% of oil spills in water after 2 h of exposure. The study's data revealed that MIL-125 could be used to photocatalyzed the cleanup of crude oil spills.
Similar content being viewed by others
Introduction
Marine oil spills typically relate to human activities that result in the release of liquid petroleum hydrocarbons into the ocean or coastal areas. They entail the release of crude oil from ships, offshore platforms, drilling rigs, and wells. Spills of crude oil can cause enormous economic losses and damage to ecological systems, public health, and communities1. Crude oil can be used not only as fuel and gasoline but also as a raw material for industrial products, such as fertilizers, pesticides, and plastics2. Dissolution, emulsification, absorption, mixing, evaporation, biodegradation, photodegradation, and chemical reactions all contribute to the degradation and dispersion of aquatic discharged oil3.
Photocatalytic oxidation is a method of converting oil into carbon dioxide, water, and salts using light as a driving energy source. Catalyzed advanced oxidation processes (AOPs) in aqueous media generate hydroxyl radicals, which are highly reactive with nonselective chemicals and have a high oxidation potential (Eo = 2.8 V) to destroy petroleum hydrocarbon molecules, allowing them to react with a wide range of oil pollutants4.
Metal-organic frameworks (MOFs) have attracted the interest of researchers because to their unusually large specific surface areas and open, interconnected microporous architectures, a high proportion of transition metals, and the capacity to be customized and modified after synthesis, and their ability to exhibit semiconductor-like behavior5,6. To date, more than 20,000 MOFs have been designed and synthesized. Most of those MOFs' applications, however, are hampered by their unstable nature. Aiming at realizing a more stable MOF, extensive efforts have been made by researchers around the world. Among the various semiconductors, TiO2 is the first example used for photocatalytic hydrogen production due to its light sensitive Ti ions. Superior to TiO2, MIL-125 not only possess Ti-oxo clusters or Ti-oxo chains/sheets, but also have light harvested ligands, endowing them with promising photocatalytic activity. The adjustable structures of MIL-125, in particular, allow them to efficiently utilise solar light beyond the ultraviolet region (which accounts for only 4% of the total)7,8,9. The stability properties of MOFs mainly contain four factors, such as chemical stability, photostability, mechanical stability, and thermal stability10,11.
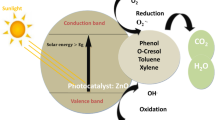
In the photocatalysis mechanism, photogenerated electrons and holes are separated and used in the same way as they are in traditional semiconductors12,13. When the photon energy is greater than or equal to the MIL-125 bandgap, the MIL-125 can be stimulated by light to generate photo-generated electrons and holes14,15. Electrons are stimulated from the highest occupied molecular orbital (HOMO) of the organic linker to its lowest unoccupied molecular orbital (LUMO). The photoexcited electrons are then passed to the MOF nodes via a linker-cluster charge transfer mechanism (LCCT), which activates the metal nodes and allows them to participate in the photooxidation reduction reaction16,17. As a result, boosting photocatalytic activity requires improvements in the quantity of generated electron–hole pairs, separation efficiency, and carrier utilization efficiency.
Direct synthesis of MIL-125 using traditional hydrothermal/solvothermal procedures is still the most widely utilized approach18. Solvents have a vital role in the crystallinity of finished goods, and the ideal solvent combination is often discovered by trial-and-error. Another important aspect that influences the shape of MOFs formed is the concentration of reactants19. Herein, we present a simple and efficient scheme to fabricate catalytic MOF by a solvothermal process. Furthermore, this study presents a novel and highly efficient photocatalytic degradation of crude oil spill using MIL-125.
Materials and methods
Materials
Terephthalic acid (H2BDC), HPLC methanol (MeOH), and anhydrous di-methyl formamide (DMF) were purchased from Fischer Scientific, UK. Titanium isopropoxide (TTIP) was produced in Sigma Aldrich, USA. Saline Water (TDS = 27,800 ppm) was taken directly from the Mediterranean Sea, Alexandria, and crude oil from the western desert, Egypt with the main characteristics shown in Table S1.
Methods
Preparation of MIL-125
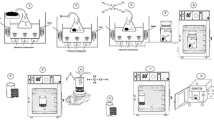
The preparation method is shown in Fig. 1; in which 6.0 mmol of terephthalic acid (H2BDC) was first dissolved into a mixed solvent of methanol (MeOH) and anhydrous dimethyl formamide (DMF) with a ratio 1:9. The solution was then treated with 1.9 mmol of titanium isopropoxide (TTIP) Ti(OCH(CH3)2)4 for 1 h while stirring. The mixture was then sonicated for 25 min and transferred into a stainless-steel autoclave system (volume: 150 mL), reacting at 150 °C for different reaction times (18, 48) h9,20,21,22. To eliminate other unreacted organic species in the mixture, the yellow solid result was washed twice with a mixed solvent of (DMF and methanol). Afterward, the solid product was isolated by centrifugation. Finally, the white powder was acquired after vacuum drying it overnight in an oven at 80 °C23. The prepared MIL-125 samples were coded (MT18, MT48).
Characterization of the prepared MOFs
Fourier transform—infrared (FT-IR)
The functional groups of MIL-125 samples were investigated using a Fourier transform—IR spectrophotometer (FTIR-8400S, Shimadzu) with a resolution of 2 cm−1. The powder samples were tested in the (4000–350) cm−1 wavenumber range.
Scanning electron microscope (SEM)
Scanning electron microscopy (SEM) (JEOL JSM 6360LA, Japan) was used to investigate the microstructure and surface morphology of all prepared samples (MT18, MT48). Before SEM imaging, the powders were sputter-coated with gold using a sputter-coating unit.
Raman analysis
A SENTERRA Raman Microscope with laser selectors (532, 633, and 785) nm was used to collect Raman spectra; the wavelength and power of the laser employed as an excitation source were 514.5 nm and 20 mW, respectively. The incident light was focused on the prepared samples and the radiation scattered by the sample was collected using a 50 objective (back-scattered configuration). A single spectrum required 360 s to acquire.
x-ray diffraction (XRD)
X-ray diffraction scans of samples were obtained using (X-ray 7000 Shimadzu-Japan) at room temperature. The degree of crystallinity was determined by applying Bragg's low where 2θ is in the range of 5°–80°, with the X-ray source being a Cu target produced at 30 kV and 30 mA and a scan speed of 4° per minute. The crystallite size is determined from the broadening of corresponding X-ray spectral peaks by the Scherrer formula24:
where D is the crystallite size (dimension), λ is the wavelength of the X-ray radiation (Cu Kaα = 0.15418 nm), K is a constant (shape factor) usually taken as 0.89, θ is the angle between the incident and reflected rays and β is the line width at half-maximum height.
Thermogravimetric analysis (TGA)
Thermogravimetric analysis is a sort of testing that is done on samples (5 mg) to assess weight loss variations as a result of temperature changes (using TGA-50 Shimadzu). This procedure was carried out at temperatures ranging from 50 to 800 °C, with a heating rate of 10 °C min−1 and a nitrogen flow rate of 20 ml min−1. This technique is used to test the prepared MOFs' thermal stability.
Surface area Brunauer, Emmett and Teller (BET)
The physical adsorption of an N2 gas on the surface of the solid and the amount of adsorbate gas corresponding to a monomolecular layer on the surface are used to calculate the specific surface area of the prepared MOFs. Physical adsorption is caused by relatively modest interactions (van der Waals forces) between adsorbate gas molecules and the test samples' adsorbent surface area. A volumetric or continuous flow approach (Quantachrome Corporation Nova 1000, version 6.11 high speed, gas sorption analyzer) at 77 K can be used to determine the amount of gas adsorbed. Before the measurements, the samples were activated at 120 °C for 6 h21,25.
Optical properties
The samples' optical characteristics were recorded to figure out where in the electromagnetic spectrum they absorb light. The UV–Vis spectrum of prepared MIL-125 was recorded using a UV–Vis spectrophotometer (T60 UV–visible) at wavelength scan ranges from 270 to 650 nm.
Photocatalytic degradation test
The prepared MOFs catalysts (MT18, MT48) were tested for the photocatalytic degradation of oil spills using mixed light sources (UV, and visible), and the experimental conditions are shown in Table S2. Each experiment was designed by adding (40 µL) crude oil to (100 mL) seawater under continuous stirring26. Firstly, the solution was kept in the dark for 30 min under continuous stirring then irradiation with UV–Vis light (a tungsten lamp of 100-W intensity as a source of visible light and four UV lamps of 2-W each as a source of UV light) was performed and the samples were taken each 30 min and then centrifuged to remove the catalysts. Samples were mixed with an equal volume of toluene with vigorous stirring for 30 min. Leave samples in a separating funnel then measure the remaining hydrocarbons in an aqueous layer by using UV-spectrophotometer at λ = 420 nm and calculate removal efficiency (RE%) using the following equation27,28
where TH Control is the oil concentration of the control and TH Treated is the oil concentration of the degraded sample.
All the photodegradation experiments were carried out in duplicate with the prepared photocatalyst. Gas Chromatography/Mass Spectrometry (GC/MS Shimazu QP2010 ultra) can help to make conclusive identification of compounds. These were the GC/MS operating conditions; the oven initial temperature was 80 °C for 0 min, raged at 10 °C min−1 to 240 °C, and then held for 2 min. The temperature was then ramped to 280 °C at 5 °C min−1, and then held at 280 °C for 10 min. The injection temperature and volume were 250 °C and 1 µL, respectively. The split ratio was 20:1. The carrier gas used was He while the solvent delay time was 2.50 min. The transfer and source temperatures were 250 °C and 150 °C, respectively. Scanning was done from 50 to 500 Da and the column dimension was 27.0 m × 250 µm.
Results and discussion
The reaction of titanium isopropoxide as a metal precursor with terephthalic acid as an organic ligand in a proper percentage of solvent mixtures (N,N-dimethylformamide (DMF), and methanol) led to well-crystallized white powder of MIL-125. MIL-125 is composed of basic units of Ti8O8(OH)4-(O2C-C6H5-CO2)6 and is built up from cyclic octamers constructed from corner or edge-sharing octahedral titanium units, as shown in Fig. S1. These octamers are connected to 12 other cyclic octamers through H2BDC linkers, leading to a porous three-dimensional quasi-cubic tetragonal structure having two types of cages, an octahedral (12.5 Å) and a tetrahedral (6 Å) cage, accessible through narrow triangular windows of ca. 6 Å25.
IR and Raman spectra in Fig. 2A,B were used to analyze the structure and morphology of the prepared samples (MT18, MT48). All IR spectra show characteristic vibrational bands in the region of (1400–1700) cm−1 for the carboxylic acid functional group of the Ti-coordinated MOF structure. Two absorption bands around (1640, and 1500) cm−1 can be assigned to carbonyl asymmetric stretching vibrations, whereas the bands at (1571, and 1628) cm−1 could be ascribed to the phenyl C=C ring stretching and the C=O stretching27. The band at 1260 cm−1 belongs to the C–H symmetric stretching vibrations of the benzene ring, which is assumed by the Raman band at 1611 cm−120. The IR region of (400–800) cm−1 shows the Ti–O–Ti–O (Ti-oxo clusters) vibrations20,21. The bending and symmetric stretching of the framework Ti–O–Ti–O octatomic ring species may be seen at Raman bands (546, 1234, and 1414) cm−1. The resonance-enhanced Raman band at 703 cm−1 is attributed to the framework titanium species in octahedral coordination settings, i.e., every single titanium atom is coupled to six oxygen atoms. The terephthalic acid ligand activates MT Raman, as evidenced by bands indexed at (1618, 1455, 1181, and 1138) cm−1, which correspond to the in-plane vibrational modes of aromatic rings, and bands centered (865 and 641) cm−1, which correspond to the vibrational modes of the aromatic rings' C–H and C=C bonds29.
XRD displays in Fig. 2C,D, where the porous crystalline carboxylate-based MIL-125 is reported in 2009 by Serre et al., which were synthesized by direct solvothermal30. Fine powders of MIL-125 were acquired and their structure was determined by synchrotron powder XRD. The MIL-125 framework, Fig. S1, is composed of cyclic Ti8O8(OH)4(COO)12 octamers linked by terephthalic linkers to an edge-and-corner-sharing TiO5(OH) octahedron. The units are arranged in a centered cubic (cc) fashion, packing into the quasi-cubic tetragonal lattice, and each octamer has 12 units neighbors. The 3D structure has two types of cages corresponding to the octahedral and tetrahedral vacancies of cc packing, with effectively accessible diameters of 12.55 Å and 6.13 Å, respectively, and connected by triangular windows of 5–7 Å18. Because the photocatalytic activity is dependent on the active sites which are also influenced by the crystallite size, which is calculated using the Debye–Scherrer equation. It is found that the crystallite size of MT48 is 7 nm which is a little smaller than for MT18 as shown in Table 1.
The nitrogen adsorption–desorption isotherms, pore diameter, and pore volume of MIL-125 catalysts are shown in Fig. 3. Table 2 shows the pore size distribution displays pores with a diameter between (4.6 and 9.2) nm, for MT48 and MT18 respectively, which correspond to the microporous and mesoporous contributions, respectively, confirming the multimodal distribution. The MT48 catalyst's BET-specific surface area and total pore volume were 301 m2 g−1 and 0.2444 cm3 g−1 respectively, showing that it is a highly porous material. More active sites would be exposed if mesopores with a bigger surface area were formed, which would boost photocatalytic activity.
SEM images Fig. 4A,B reveal that both (MT18, MT48) samples are made up of particles with typical circular plate-like morphology9 with particle sizes (258 ± 89, 234 ± 65) nm, respectively. The thermogravimetric analysis (TGA), in Fig. 4C, indicates a steady mass loss of the prepared MOFs before 800 °C. The first weight loss before 200 °C at (80 °C, 61 °C) with weight loss (3.6%, 2.6%) for (MT18, MT48), respectively. This may be to the elimination of physically adsorbed gases, moisture, and DMF from the pores presumably owing to solvate evaporation31. The second weight loss between (200–500) °C is due to the framework disintegration32, which eventually produces amorphous TiO2 residue33. The residues were (75.33, and 49) % of the initial mass for both (MT18, and MT48), respectively.
Optical characterization
The optical properties of prepared MOFs (MT18, MT48) were investigated using the UV–VIS spectrum which is shown in Fig. 5A. Due to the octahedral coordination in titanium species, the two prepared samples (MT18, MT48) possess a broad absorption edge at wave length (310 nm), MT18, MT48 absorb only at wavelength below 310 nm, inducing excitation from HOMO, made of π states in the ligand and O(2p) in the metal-oxo cluster, into the LUMO defined by Ti(3d), leaving behind holes near the Ti-oxo cluster9,34,35. The optical bandgap (Eg) demonstrates a relationship between the absorption coefficient and the incident photon energy and can be calculated by using the following Tauc’s equation35,36.
where α is the coefficient of the absorption, hυ is the incident photon energy, A is a constant, Eg represents the bandgap, and n is determined based on the type of optical transition of prepared MOFs.
These materials have an indirect optical transition, thus n = 2. The optical energy gap (Eg) can be deduced from plotting the relationship between (αhʋ)1/2 and photon energy (hʋ) for MT18 and MT48 as shown in Fig. 5B. The calculated Eg of MT18 and MT48 is ∼ 4.03 eV and ∼ 3.2 eV, respectively, from the intercept of the tangent to the line. The reduction of band gap values from 4.03 eV for MT18 to 3.2 eV for MT48 may be due to the particle size decreasing from (258 to 234) nm as the solvothermal reaction time increase from (18 to 48) h37.
Photocatalytic degradation of crude oil spills
Figure 6 shows the photo-degradation study of (crude oil/saline water) samples by using different MOF catalysts and it clearly shows that the disappearance of oil spills after photocatalytic reaction using MT18 and MT48. The concentration of crude oil before and after photodegradation can be determined using UV–Vis spectrophotometry and GC/MS technique. Figure 5 shows the UV–Vis spectrophotometry of degraded crude oil with respect to using the light as controlled value (absorbance = 3.344). Generally, the two prepared MIL-125 samples display a high efficiency toward the degradation of crude oil. Figure 7A shows that the use of different doses of MT18 as photocatalyst (125, 250, and 500) ppm in the degradation of crude oil spill revealed high degradation efficiencies reached 98.4% after 2 h of irradiation. On the other hand, Fig. 7B shows that using 250 ppm of MT48 as photocatalyst can degrade about 99% of crude oil spills after 2 h of irradiation. The MT48 has little more degradation efficiency under (UV–Vis light) this is due to the lower optical band gap (3.2 eV) and higher surface area (301.1 m2 g−1)37. The trend observed in the UV–Vis spectrophotometry analysis is agreed with the optical properties and morphological characterization results of prepared photocatalyst.
The GC/MS chromatograms of the analyzing wastewater samples before and after photodegradation are presented in Fig. 8. This analysis shows that water contaminated with crude oil spills (Control) contains different compounds such as Pentane-2-methyl, Tetrahydrofuran (THF), Cyclohexane, 6-Bromo-4-(2-choro-phenyl)-2-piperan, Benzene, (1-butylhexadecl), Benzene, (1-methylnonyl), Benzene, (1-buylheptyl), Benzene, (1-propyloctyl), Octadecane and Benzene, (1-pentylheptyl). The obtained chromatograms after photocatalytic degradation using MT18 and MT48 cleared that all mentioned compounds are degraded with high efficiencies and their concentrations become nil38.
Kinetics of the reaction rate
Under various experimental conditions, our findings indicated that MIL-125 could be used to degrade marine oil spills. The photoreaction process can be divided into two stages based on the kinetic reaction curves in Fig. 9. First, the concentration decreased rapidly at the start of the irradiation, and the photoreaction confirmed a first-order kinetic pattern. Secondly, the concentration was nearly stabilized. According to the first-order kinetic reaction:
where Co is the initial reaction concentration of oil; Ct is the concentration of oil measured at a fixed time; t is irradiation time, and \(K\) is is the reaction rate determined by the linear plot of In (Co/Ct) versus the time. Table 3 summarizes the kinetic data from this study's photo-degradation of oil under various experimental conditions. According to our results, photochemical degradation is an effective method for removing petroleum hydrocarbons from seawater.
Conclusions
From this research, it is possible to conclude that, efficient MIL-125 photocatalysts have been successfully prepared via a facile solvothermal method. The sample prepared at 150 °C and a reaction time of 48 h (MT48) has a low crystal size of 7 nm and a particle size of 234 nm with an optical bandgap of 3.2 eV and a surface area of 301 m2 g−1. Based on GC/MS and UV/Vis photodegradation results, the two prepared samples show high efficiency toward the degradation of crude oil. And the use of 250 ppm of MT48 photocatalyst under UV–Vis irradiation can degrade about 99% of oil spills in water. This study presents a novel and highly efficient photocatalytic degradation of crude oil spills using MIL-125.
Data availability
The generated and analyzed data during the current study supplied in this manuscript and is readily available from the corresponding author upon reasonable request.
Change history
20 February 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-30138-5
References
Zhang, B. et al. Marine Oil Spills-Oil Pollution, Sources and Effects 2nd edn. (Elsevier, 2018).
Luo, C. et al. Efficient biodegradation of phenanthrene using Pseudomonas stutzeri LSH-PAH1 with the addition of sophorolipids: Alleviation of biotoxicity and cometabolism studies. Environ. Pollut. 301, 119011. https://doi.org/10.1016/j.envpol.2022.119011 (2022).
King, S. M. et al. Photolytic and photocatalytic degradation of surface oil from the Deepwater Horizon spill. Chemosphere 95, 415–422. https://doi.org/10.1016/j.chemosphere.2013.09.060 (2014).
Shivaraju, H. P., Muzakkira, N. & Shahmoradi, B. Photocatalytic treatment of oil and grease spills in wastewater using coated N-doped TiO2 polyscales under sunlight as an alternative driving energy. Int. J. Environ. Sci. Technol. 13, 2293–2302. https://doi.org/10.1007/s13762-016-1038-8 (2016).
Assi, H. et al. Titanium coordination compounds: From discrete metal complexes to metal-organic frameworks. Chem. Soc. Rev. 46, 3431–3452. https://doi.org/10.1039/c7cs00001d (2017).
Chang, Y. J. et al. Ultrahigh efficient laser desorption ionization of saccharides by Ti-based metal-organic frameworks nanosheets. Anal. Chim. Acta 1032, 91–98. https://doi.org/10.1016/j.aca.2018.06.035 (2018).
Hu, N. et al. Amino-functionalized titanium based metal-organic framework for photocatalytic hydrogen production. Molecules https://doi.org/10.3390/molecules27134241 (2022).
Li, L., Wang, X. S., Liu, T. F. & Ye, J. Titanium-based MOF materials: From crystal engineering to photocatalysis. Small Methods 4, 1–28. https://doi.org/10.1002/smtd.202000486 (2020).
Wang, J. et al. Ti-based MOFs: New insights on the impact of ligand composition and hole scavengers on stability, charge separation and photocatalytic hydrogen evolution. Appl. Catal. B Environ. 283, 119626. https://doi.org/10.1016/j.apcatb.2020.119626 (2021).
Wang, X. S., Li, L., Li, D. & Ye, J. Recent progress on exploring stable metal-organic frameworks for photocatalytic solar fuel production. Sol. RRL 4, 1–34. https://doi.org/10.1002/solr.201900547 (2020).
Photocatalytic, F. M. Achievements and perspectives in metal—organic nitrogen reduction (2022).
Gao, J. et al. A p–type Ti(iv)–based metal–organic framework with visible–light photo–response. Chem. Commun. 50, 3786–3788. https://doi.org/10.1039/c3cc49440c (2014).
Assi, H. et al. Investigating the case of titanium(IV) carboxyphenolate photoactive coordination polymers. Inorg. Chem. 55, 7192–7199. https://doi.org/10.1021/acs.inorgchem.6b01060 (2016).
Dhakshinamoorthy, A., Asiri, A. M. & García, H. metal-organic framework (MOF) compounds: Photocatalysts for redox reactions and solar fuel production. Angew. Chem. Int. Ed. 55, 5414–5445. https://doi.org/10.1002/anie.201505581 (2016).
Sun, D. & Li, Z. Robust Ti- and Zr-based metal-organic frameworks for photocatalysis. Chin. J. Chem. 35, 135–147. https://doi.org/10.1002/cjoc.201600647 (2017).
Wang, C. C., Yi, X. H. & Wang, P. Powerful combination of MOFs and C3N4 for enhanced photocatalytic performance. Appl. Catal. B Environ. 247, 24–48. https://doi.org/10.1016/j.apcatb.2019.01.091 (2019).
Fu, Y. et al. An amine-functionalized titanium metal-organic framework photocatalyst with visible-light-induced activity for CO2 reduction. Angew. Chem. Int. Ed. 51, 3364–3367. https://doi.org/10.1002/anie.201108357 (2012).
Zhu, J. et al. Titanium-based metal–organic frameworks for photocatalytic applications. Coord. Chem. Rev. 359, 80–101. https://doi.org/10.1016/j.ccr.2017.12.013 (2018).
Chen, X. et al. Recent advances in titanium metal–organic frameworks and their derived materials: Features, fabrication, and photocatalytic applications. Chem. Eng. J. 395, 125080. https://doi.org/10.1016/j.cej.2020.125080 (2020).
Hu, S. et al. Solvothermal synthesis of NH2-MIL-125(Ti) from circular plate to octahedron. CrystEngComm 16, 9645–9650. https://doi.org/10.1039/c4ce01545b (2014).
Wen, G. & Guo, Z. G. Facile modification of NH2-MIL-125(Ti) to enhance water stability for efficient adsorptive removal of crystal violet from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 541, 58–67. https://doi.org/10.1016/j.colsurfa.2018.01.011 (2018).
Wang, S. et al. Toward a rational design of titanium metal-organic frameworks. Matter 2, 440–450. https://doi.org/10.1016/j.matt.2019.11.002 (2020).
Kaur, M., Mehta, S. K. & Kansal, S. K. Amine-functionalized titanium metal-organic framework (NH2-MIL-125(Ti)): A novel fluorescent sensor for the highly selective sensing of copper ions. Mater. Chem. Phys. 254, 123539. https://doi.org/10.1016/j.matchemphys.2020.123539 (2020).
Drits, V., Środoń, J. & Eberl, D. D. XRD measurement of mean crystallite thickness of illite and illite/smectite: Reappraisal of the Kubler index and the Scherrer equation. Clays Clay Miner. 45, 461–475. https://doi.org/10.1346/CCMN.1997.0450315 (1997).
Kim, S. N. et al. Adsorption/catalytic properties of MIL-125 and NH2-MIL-125. Catal Today 204, 85–93. https://doi.org/10.1016/j.cattod.2012.08.014 (2013).
Li, Y., Zhang, Q., Jiang, J. & Li, L. Long-acting photocatalytic degradation of crude oil in seawater via combination of TiO2 and N-doped TiO2/reduced graphene oxide. Environ. Technol. https://doi.org/10.1080/09593330.2019.1647291 (2019).
Zhang, S. et al. Defect-rich and electron-rich mesoporous Ti-MOFs based NH2-MIL-125(Ti)@ZnIn2S4/CdS hierarchical tandem heterojunctions with improved charge separation and enhanced solar-driven photocatalytic performance. Appl. Catal. B Environ. 262, 118202. https://doi.org/10.1016/j.apcatb.2019.118202 (2020).
El-Borai, A. M., Eltayeb, K. M., Mostafa, A. R. & El-Assar, S. A. Biodegradation of industrial oil-polluted wastewater in Egypt by bacterial consortium immobilized in different types of carriers. Pol. J. Environ. Stud. 25, 1901–1909. https://doi.org/10.15244/pjoes/62301 (2016).
Hlophe, P. V. & Dlamini, L. N. Synthesis of a semi-conductor-like MOF with black phosphorous as a composite for visible light-driven photocatalysis. RSC Adv. 9, 37321–37330. https://doi.org/10.1039/c9ra08296d (2019).
Meenakshi, D.-H. et al. A new photoactive crystalline highly porous titanium(IV) dicarboxylate. J. Am. Chem. Soc. 131, 25 (2009).
Chao, M. Y., Zhang, W. H. & Lang, J. P. Co2 and Co3 mixed cluster secondary building unit approach toward a three-dimensional metal-organic framework with permanent porosity. Molecules https://doi.org/10.3390/molecules23040755 (2018).
Peng, M. M. et al. Oxidation of ethylbenzene using nickel oxide supported metal organic framework catalyst. Bull. Korean Chem. Soc. 35, 3213–3218. https://doi.org/10.5012/bkcs.2014.35.11.3213 (2014).
Hu, S. et al. Surfactant-assisted synthesis of hierarchical NH2-MIL-125 for the removal of organic dyes. RSC Adv. 7, 581–587. https://doi.org/10.1039/c6ra25745c (2017).
Sun, D., Ye, L. & Li, Z. Visible-light-assisted aerobic photocatalytic oxidation of amines to imines over NH2-MIL-125(Ti). Appl. Catal. B Environ. 164, 428–432. https://doi.org/10.1016/j.apcatb.2014.09.054 (2015).
Capano, G. et al. On the electronic and optical properties of metal-organic frameworks: Case study of MIL-125 and MIL-125-NH2. J. Phys. Chem. C 124, 4065–4072. https://doi.org/10.1021/acs.jpcc.9b09453 (2020).
Mostafa, M., El Nady, J., Ebrahim, S. M. & Elshaer, A. M. Synthesis, structural, and optical properties of Mn2+ doped ZnS quantum dots for biosensor application. Opt. Mater. (Amst.) 112, 110732. https://doi.org/10.1016/j.optmat.2020.110732 (2021).
Ghosh, R. et al. The effect of cluster size on the optical band gap energy of Zn-based metal-organic frameworks. Dalt. Trans. 44, 13464–13468. https://doi.org/10.1039/c5dt02132d (2015).
Agyei-tuffour, B., Gbogbo, S., Dodoo-arhin, D., et al (2020) Photocatalytic degradation of fractionated crude oil: Potential application in oil spill remediation Photocatalytic degradation of fractionated crude oil: Potential application in oil spill remediation. https://doi.org/10.1080/23311916.2020.1744944.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization, writing, methodology, validation, formal analysis, and data curation, M.S.S.; writing, review and editing, A.M.A.E.-A., R.R. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in Affiliation 2, which was incorrectly given as ‘Department of Chemistry, College of Science, University of Jeddah, Jidda, Saudi Arabia’. The correct affiliation is: Department of Chemistry, College of Science, University of Jeddah, Jeddah, Saudi Arabia.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Showman, M.S., El-Aziz, A.M.A. & Yahya, R. Efficient photocatalytic degradation of petroleum oil spills in seawater using a metal-organic framework (MOF). Sci Rep 12, 22445 (2022). https://doi.org/10.1038/s41598-022-26295-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26295-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.