Abstract
Potassium-ion batteries (KIBs) are emerging as attractive alternatives to lithium-ion batteries for the large scale energy storage and conversion systems, in view of the natural abundance and low cost of potassium resources. However, the lack of applicable anodes for reversible accommodation to the large K+ limits the application of KIBs. Herein, porous Sb-graphene-carbon (Sb-G-C) nanofibers are fabricated via a scalable and facile electrospinning approach. As an attempt, the nanofibers weaving into flexible mats are introduced as binder-free anode materials of KIBs, presenting a great cycle life (204.95 mAh g−1 after 100 cycles at 100 mA g−1), as well as the excellent rate capability (120.83 mAh g−1 at 1 A g−1). The superior performances of the Sb-G-C anodes can be derived from the dispersed graphene, which offers enhanced tolerance to the volume change and promotes the electron transportation, accounting for the outstanding cyclability and rate capability. Furthermore, the extrinsic pseudocapacitance created from the 1D porous nanostructure of the Sb-G-C also boosts the K+ storage capacity. The presented results may pave a new pathway for future high-performance KIBs.
Export citation and abstract BibTeX RIS
1. Introduction
Due to the vast consumption of fossil fuels, the development of environmentally friendly and low cost sustainable energy is urgently needed, and lithium-ion batteries (LIBs) have been regarded as a key technology for power storage [1–3]. However, strained lithium resources make the state-of-the-art LIBs more expensive, which inhibit the long-term application of LIBs to smart grids [4]. Recently, alternative alkali-ion batteries with abundantly natural resources concurrently showing similar 'rocking chair' electrochemical behaviors, including sodium-ion batteries (SIBs) and potassium-ion batteries (KIBs), have attracted significant research interest [5]. Indeed, the redox potential of K/K+ couple (−2.92 V vs SHE) is closer to Li/Li+ (−3.04 V vs SHE) than that of Na/Na+ (−2.71 V vs SHE), meriting KIBs with potentially wider electrochemical voltage window and higher energy density compared to SIBs [6–9]. With the exploration of high-performing cathode and anode materials for KIBs, the full cells could achieve great energy densities of 58.2–100.7 Wh kg−1 [10–12]. Actually, in kinds of organic electrolytes, such as ethylene carbonate (EC) /diethyl carbonate (DEC), the K/K+ even exhibits lower redox potential than Li/Li+ due to the weaker reaction with solvent molecules [5]. Besides, the lower charge density of K+ in liquid electrolytes also results in smaller Lewis acidity and desolvation energy, which benefit the fast transportation and intercalation kinetics [8]. Another advantage of KIBs is that the potassiation potential (0.2 V vs K/K+) is more positive than the lithiation (0.1 V vs Li/Li+) and sodiation potential (0.05 V vs Na/Na+) [13], which might reduce the risk of metal plating/stripping and avoid the formation of potassium dendrites during cycling, making KIBs safer than LIBs and SIBs [5, 14]. Early publications have shown that the cheaper aluminum current collector can be applied in KIBs to support the anode materials because potassium cannot alloy with aluminum even at low potentials [5, 15]. These promising facts make KIBs an attractive alternative to replace LIBs. However, the large-sized K+ (Li+ < Na+ < K+, 0.76 < 1.02 < 1.38 Å) plagues the electrochemical stability of electrode materials for KIBs [15–17]. The large volume expansion suffered by the active materials during K+ insertion/extraction readily leads to severe structural deterioration and then the loss of electrical contact with the current collector, greatly impacting the cyclability and capacity for potassium storage.
Among the studies focused on advanced KIBs anodes, typical carbonaceous materials with different properties (hard carbon [5, 18], soft carbon [19, 20], hard-soft composite carbon [21], and graphite [22–24]), as well as different morphologies (carbon microspheres [18], carbon nanotubes [25], carbon nanocage [26], and reduced graphene oxides [27–29]), have been successfully applied. Nonetheless, great efforts are still being dedicated to developing carbon-based electrodes with higher performances for K+ storage. Previous reports have shown that metallic Sb can be considered to be a promising anode material for KIBs owing to its environmental benignity, low cost (∼\$10 kg−1), and high theoretical capacity (660 mAh g−1 with K3Sb) [30]; but a 407% volume expansion from Sb to K3Sb will make the active materials prone to pulverization [30–33]. McCulloch et al have first introduced the Sb-carbon (Sb-C) composites for K+ storage, further presenting that the capacity decay links to a mechanical disintegration [30]. To date, most studies about Sb-C electrodes applied in LIBs/SIBs/KIBs have addressed that optimizing the Sb to nanostructures and dispersing the Sb nanoparticles in an appropriate carbon host is a prospective strategy [30, 33–35]. Besides, experiences received from our earlier research devoting to exploring high-performance KIB anodes show that modifying the substrate with certain highly conductive materials, such as graphene, is also an effective measure for tackling the swelling pressure problem [31, 36]. These findings could enlighten us about the structural design of advanced KIB anodes.
Latest progress in binder-free electrodes for energy storage devices with lightweight and self-standing ability has brought new opportunities for the development of KIBs. However, only a few literature reports are available for flexible KIB anodes [37–40]. Compared with electrodes made by slurry-casting, a flexible electrode with bind-free configuration can be directly adopted, so as to perfectly eliminate the demand for binder/conductive additive, realize robust electrical contact with its highly conductive matrix, and accelerate ion diffusion, providing higher energy density [38, 40]. In this paper, we were motivated to probe into the fabrication of Sb-graphene-carbon (Sb-G-C) nanofibers via a facile electrospinning, and their anode application for KIBs. The prepared flexible Sb-G-C with free-standing membrane functioned as current collector- and binder-free anode directly, which exhibited a stable cycle life of 204.95 mAh g−1 at 100 mA g−1 after 100 cycles (capacity decay rate of ≈0.31% per cycle), and high rate capability of 120.83 mAh g−1 at 1 A g−1 in contrast to the Sb-C counterpart, certifying the improved potassium storage performances by graphene modification. The dispersed graphene inside nanofibers can not only further mitigate the stress induced by volume expansion, but also improve the electrical conductivity of the active materials. Additionally, by investigating the K+ storage mechanism of Sb-G-C anodes via electrochemical kinetics analysis, it is discovered that the pseudocapacitive effect originated from its porous nanostructure is identified as the major contribution to the energy storage process, leading to an extended cycling stability and fast K+ uptakes. In consideration of the outstanding electrochemical characteristics and facile synthesis, the Sb-G-C nanofibers possess enormous potential as flexible anode materials for K-storage application.
2. Experimental details
Preparation of the Sb-G-C nanofibers: The graphene oxide (GO) was produced from natural flaky graphite by a modified Hummers method [41]. After centrifugation and washing-up for many times, the homogeneous GO was finally collected and dispersed in N, N-dimethylformamide (DMF). Briefly, 0.251 g SbCl3 (Sigma-Aldrich), 0.408 g polyacrylonitrile (PAN, Mw = 150 000 g mol−1, Sigma-Aldrich) were dissolved in 6 ml DMF, followed by the dropwise addition of 0.1 ml GO/DMF dispersion (0.4 mg ml−1). Through vigorous magnetic stirring at 60 °C, the electrospinning was performed at room temperature and humidity. Subsequently, the as-collected white film consisting of entangled nanofibers was treated in air at 210 °C for 6 h with a low heating-up rate of 1 °C min−1, and finally pyrolyzed at 2 °C min−1 to 700 °C for 2 h under a mixed atmosphere of 10% H2/Ar in a tubular furnace. In this case, PAN was partially decomposed into gas while carbonized to the carbon skeleton, accompanied with the reduction of SbCl3 to Sb, and GO to graphene under H2/Ar protection, forming the Sb-G-C nanofibers. For comparison, Sb-C nanofibers without GO and Sb-G-C counterparts incorporating 0.05 and 0.2 ml GO/DMF in the precursor solution were also synthesized using the same method.
Structural characterization: The microstructure and morphology of the obtained specimens were observed by scanning electron microscopy (SEM, Hitachi S4800) and transmission electron microscopy (TEM, FEI Tecnai-F20). The crystal structure was characterized by x-ray diffraction (XRD) on a Rigaku Desktop x-ray diffractometer using Cu Kα radiation (λ = 0.154 nm) over a 2θ region between 10° and 80°. X-ray photoelectron spectroscopy (XPS) was recorded on a Surface Science Instruments S-probe spectrometer. Thermogravimetric analysis (TGA) was performed in air atmosphere under a heating rate of 10 °C min−1 from room temperature to 750 °C, for evaluating the Sb content (PerkinElmer, Diamond TG analyzer). A Belsorp-Mini analyzer apparatus was applied to measure the N2 adsorption/desorption properties and the pore size distribution of the prepared Sb-G-C.
Electrochemical measurements: The electrochemical performances of the products were examined by constructing CR2025 coin-type cells. The half-cells were assembled in an Ar-filled glovebox, with both oxygen and water contents below 0.5 ppm, according to our earlier reported work [42–44]. The flexible nanofiber mats (Sb-G-C or Sb-C) were cut into square pieces, which were directly assembled into cells without adding polymeric binder or conductive agent. The average mass loading for both Sb-G-C and Sb-C was about 0.9 mg cm−2. The counter electrode was K metallic foil, and the separator was a glass microfiber filter (Whatman). The electrolyte solution was 0.8 M KPF6 dissolved in a 1:1 (v/v) mixture of EC/DEC. Galvanostatic charge/discharge tests were conducted on an Arbin BT2000 system at room temperature, with a potential range of 0.01–2.5 V. Cyclic voltammetry (CV) was accomplished using a CHI660E electrochemical workstation (0.01–3 V).
3. Results and discussion
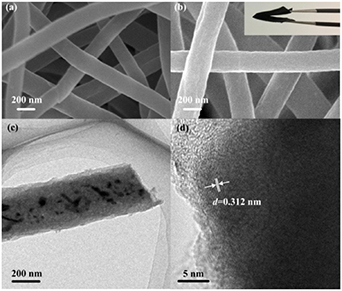
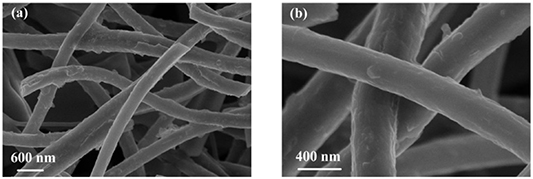
Morphologically, SEM images of the as-prepared Sb-C and Sb-G-C composites were presented in figure 1, indicating the continuous 1D framework for both Sb-C and Sb-G-C. It can be found that little apparent morphological difference has been induced by graphene modification except for an average diameter of about 225 nm for Sb-C (figure 1(a)) while 265 nm for Sb-G-C (figure 1(b)). Meanwhile, the Sb-G-C nanofibers exhibit good mechanical flexibility and can be repeatedly bent without breaking (inset in figure 1(b)). The TEM image in figure 1(c) shows that the metallics are well encapsulated in the carbon matrix, and mesopores can be clearly observed on the Sb-G-C nanofiber surface at higher magnification (figure S1 (available online at https://stacks.iop.org/NANO/32/025401/mmedia)). Figure 1(d) illustrates the lattice fringes of the embedded nanoparticles characterized by high-resolution TEM (HRTEM), where the d-spacing measured to be 0.312 nm from the peak position is ascribed to the (012) lattice plane of the hexagonal Sb. In addition, as the graphene finely disperses throughout the 1D nanofibers and owns a low mass ratio (<0.1 wt%), there is no obvious graphene observed in TEM and HRTEM images.
Figure 1. SEM images of (a) Sb-C and (b) Sb-G-C (inset: digital photo of flexibility demonstration), (c) TEM and (d) HRTEM images of Sb-G-C.
Download figure:
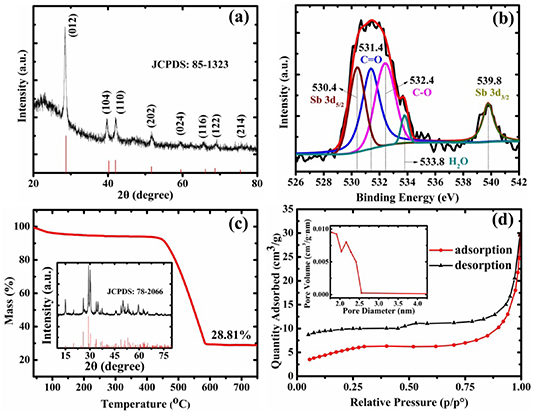
Standard image High-resolution imageFigure 2(a) displays the XRD pattern of the prepared Sb-G-C nanofibers, and indicates that all the diffraction peaks could be indexed to the hexagonal Sb (JCPDS card no. 85-1323), further confirming the encapsulated Sb particles in the nanofibers. According to Scherrer's formula in equation (1):
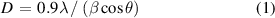
Figure 2. (a) XRD pattern, (b) XPS results of the Sb 3d, (c) TG analysis from 40 °C to 800 °C in air (inset: XRD pattern of the combustion products), and (d) nitrogen adsorption/desorption isotherms (inset: pore size distribution) of the Sb-G-C.
Download figure:
Standard image High-resolution imagewhere D is the crystallite size, λ is the x-rays wavelength, β is the full-width at half-maximum (FWHM) and θ is the Bragg angle, the average crystallite size of the embedded Sb in Sb-G-C is calculated to be ca. 16.65 nm, while the mean crystal size of the Sb phase in the Sb-C sample estimated from its XRD pattern (figure S2(a)) is ca. 16.19 nm, illustrating that the Sb particles crystallization is not apparently affected by graphene modification. XPS analysis was conducted to identify the valence states of the atoms on the surface of the Sb-G-C. As the Sb 3d spectrum shown in figure 2(b), the spin orbit split Sb 3d3/2 located at 539.8 eV was detected, demonstrating the existence of Sb element in the nanofibers. Besides, due to the highly overlapped binding energy between the Sb 3d5/2 and the O 1 s spectrum, the main peak between 529 and 535 eV could be deconvoluted into four branch signals at 530.4, 531.4, 532.4 and 533.8 eV, which respectively correspond to the Sb 3d5/2, C = O, C–O and H2O [45–47].
To examine the Sb content in the Sb-G-C nanofibers, TGA was carried out under air flow, with a heating rate of 10 °C min−1. From the TGA result shown in figure 2(c), only 4.96% weight loss is observed below 180 °C, mainly resulted from the evaporation of absorbed water. When the temperature increases to 600 °C, a major weight loss of more than 60% is attributed to the oxidation of the carbon matrix and graphene into gaseous CO2. The final residue of 28.81% in weight after TGA is affirmed to be Sb2O4 (inset in figure 2(c)), thus the calculated amount of Sb is 22.81% based on equation (2):
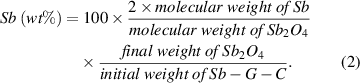
To gain a better investigation about the porous property of the obtained Sb-G-C, a nitrogen adsorption/desorption measurement was applied (figure 2(d)). The isotherms are revealed to be close to type IV pattern, implying the mesoporous structural features of the nanofibers. According to the Brunauer–Emmett–Teller (BET) method, the Sb-G-C possesses a specific surface area of 19.67 m2 g−1. Meanwhile, the pore size mostly lies between 2 and 4 nm based on the Barrette–Joynere–Halenda (BJH) model (inset in figure 2(d)), with a pore volume of 0.046 cm3 g−1, confirming that uniform surface pores have been fabricated on the Sb-G-C nanofibers by annealing treatment. It has been well known that the porous nanostructure accommodates the volume fluctuation upon cycling, concurrently favors the accessibility of the active material to the electrolyte and ensures rapid K+ diffusion kinetics [48]. Compared with the BET results of Sb-C counterpart showing the specific surface area of 5.71 m2 g−1, as well as a total pore volume of 0.014 cm3 g−1 (figure S2(b)), it is obvious that after the incorporation of graphene, the Sb-G-C nanocomposites display increasing BET surface area and total pore volume.
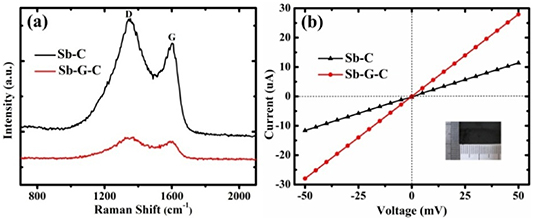
Raman spectrum was recorded to clarify the graphitization of carbonaceous materials. For Sb-G-C, as depicted in figure 3(a), typical bands at 1373 and 1589 cm−1 are assigned to the vibration of disordered carbon (D band) and the in-plane sp2 carbon atoms (G band), respectively [49, 50]. The relative intensity ID/IG for Sb-G-C composites (1.01) is lower than that of Sb-C (1.07), verifying an increased graphitic crystalline structure due to the introduction of graphene. For further investigation, the positive effect produced by the graphene modification on electrical conductivity was also explained, by linear sweep voltammetry with an Agilent 4156 semiconductor parameter analyzer. The Sb-C and Sb-G-C flexible mats tailored into rectangular slices (21 × 11 mm2) were clamped and connected to the probe station. As denoted in figure 3(b), with the voltage sweeping forward from −50 to 50 mV, current-voltage (I–V) characteristics were taken down. Afterwards, electrical conductance of the Sb-C and Sb-G-C nanofiber mats can be respectively calculated as 0.23 × 10−3 and 0.56 × 10−3 S, suggesting that the outstanding electrical conductivity of graphene makes the Sb-G-C an ideal electrode for the efficient, fast and continuous transportation of charge carriers within the fibrous frameworks [51–53].
Figure 3. (a) Raman spectra, and (b) electrical conductivity tests of the Sb-C and Sb-G-C nanofibers.
Download figure:
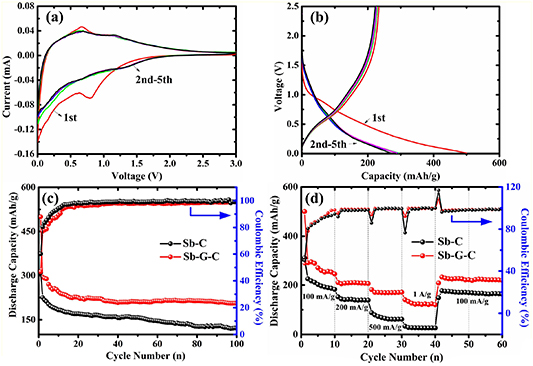
Standard image High-resolution imageAs can be seen in figure 4(a), the K+ storage reactivity of the Sb-G-C was first investigated by CV curves (0.1 mV s−1). In the initial cycle, a small cathodic peak at about 0.8 V denotes the irreversible decomposition of the electrolyte and formation of solid electrolyte interface (SEI) film. For the following cathodic scans, a major reduction peak near 0.01 V can be observed, resembling the behavior of K+ insertion into carbon; while two weak reduction peaks at 0.63 V and 0.12 V may be assigned to the alloying reaction between Sb and K, to form KSb and finally K3Sb alloy phase [30, 35]. Likewise, during the reversed anodic branches, two oxidation peaks at around 0.72 V and 1.22 V intrinsically correspond to the two-step depotassiation processes of K3Sb to generate KSb phase, and then KSb to form Sb, respectively [54, 55]. From the second cycle, both cathodic and anodic profiles have a good overlap, testifying the high reversibility and excellent K+ storage stability. Figure 4(b) depicts the galvanostatic charge/discharge profiles of the Sb-G-C for the foremost 5 cycles at 100 mA g−1. Consistent with CV results, the curves show a slightly sloping plateau between 1.0 V and 0.85 V for the first discharge, implying the SEI film formation. Moreover, two pairs of charge/discharge plateaus at about 0.6/0.2 V and 1.1/0.7 V are associated with the dealloying and alloying reactions of K-Sb. The electrode affords the first discharge (potassiation) capacity of 499.89 mAh g−1, with a relatively low initial coulombic efficiency (ICE) of 46.84%. The first cycle irreversible loss is probably caused by the reductive decomposition reaction of the electrolyte and the formation of the SEI layer on the surface of the Sb-G-C anode, which consumes K+ but buffers the swelling stress of the electrode [56]. After a few cycles, the CE of Sb-G-C increases rapidly and approximately approaches 100%. The low ICE may be a common drawback existing in most of the nanocomposites with porous structure, which brings more interfaces between the anode materials and electrolyte, and it needs more time to complete the SEI formation [57–59]. According to early reports, for diminishing the low ICE impact of the electrodes on making full cells, simple prepotassiation measure could be taken as an efficient strategy [60–62].
Figure 4. (a) CV curves at 0.1 mV s−1 and (b) galvanostatic charge/discharge profiles at 100 mA g−1 of the Sb-G-C; (c) cycling performances, and (d) rate capacities of the Sb-C and Sb-G-C electrodes.
Download figure:
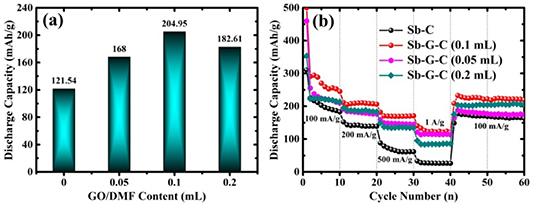
Standard image High-resolution imageThereafter, in order to compare the K+ storage properties of Sb-C and Sb-G-C electrodes, further studies about cyclability (100 mA g−1) and rate capability were elucidated (figures 4(c)–(d)). In order to compromise the charge capacity and cycle life, the cells were cycled in the potential window of 0.01–2.5 V [63–65]. The Sb-G-C retains a capacity of 204.95 mAh g−1 at the 100th cycle, contrastively for Sb-C only 121.54 mAh g−1 can be achieved over 100 cycles. The decay rates are calculated to be 0.31% for Sb-G-C and 0.46% for Sb-C per cycle relative to the 2th cycle (Sb-G-C: 294.36 mAh g−1, Sb-C: 222.25 mAh g−1), respectively. The improvements of reversible capacity and cycling stability of Sb-G-C are mainly due to the modified graphene, which is capable of enhancing the mechanical flexibility and structural integrity of the anodes. Moreover, cycling performance of Sb-G-C for longer cycle periods was also examined (figure S3), exhibiting the capacity of 163.15 mA g−1 after 200 cycles (decay rate ≈0.22% per cycle). As presented in figure 4(d), as the current rate is stepwise increased to 200, 500 and 1000 mA g−1 for every ten successive cycles, the Sb-G-C respectively presents the discharge capacities of 206.17, 170.83 and 120.83 mAh g−1, while Sb-C only allows 138.72, 61.39 and 26.12 mAh g−1, correspondingly. Such a super rate capability of the Sb-G-C firstly benefits from its porosity of the carbon framework, making the Sb nanoparticles readily accessible to the electrolyte and fully utilized. Furthermore, the graphene helps to enhance the rate capability by fastening the reversible insertion/extraction and transportation of K+. Compared with the reported Sb-C-based composites for KIBs in recent years listed in table S1, the Sb-G-C nanofibers deliver competitive electrochemical performances [30, 32, 33].
Characterization of the self-supporting Sb-G-C electrodes after long-time cycling was provided in figure 5. From the magnified SEM micrographs, it can be observed that most nanofibers still keep their original appearance after 200 cycles, testifying the function stability of the Sb-G-C anodes. The coated carbon layer and the dispersed graphene play the roles of protecting the embedded nanoparticles during K+ intercalation and deintercalation, thus retaining acceptable structural integrity and cyclic durability. For contrast, SEM images of the Sb-C nanofibers after 100 cycles have also been displayed (figure S4), highlighting the enhanced mechanical flexibility by graphene modification. Although the Sb-C sample also exhibits well macroscopic flexibility, the microstructure destruction has generated on parts of the nanofibers, associated with the specific capacity fading.
Figure 5. SEM images after 200 cycles of the Sb-G-C electrodes.
Download figure:
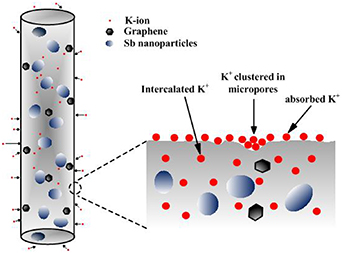
Standard image High-resolution imageFurther studies about the electrochemical kinetics of the Sb-G-C and Sb-C electrodes towards K+ were conducted by CV experiments at different sweep rates (figures S5–S6), highlighting the motivation of graphene on pseudocapacitance. As the K+ storage mechanism of Sb-G-C illustrated in figure 6, there are probably three kinds of active sites responsible for the K+ uptakes: the defect or edge sites, the micropores and the intercalation sites. The dominant pseudocapacitive behavior mainly refers to the reversible K+ absorption reaction by inserting into the surface defects or clustering in the micropores, and hence it is favorable to a fast kinetic property of the carbon-based materials upon cycling without severe deterioration. Based on the above analysis, the explanation for the excellent potassium storage capability of the Sb-G-C nanofibers can be ascribed to the morphology features. The strong interfacial interaction between the encapsulated Sb particles and a uniformly coated carbon matrix effectively maintains the structural stability. More importantly, the dispersed graphene affords flexible buffer to further accommodate the K+ insertion/extraction, concurrently provides more facile transport channels and increased diffusion efficiency for K+ and electrons. Beyond that, host anode materials with porous nanostructure could induce obvious pseudocapacitive characteristics by promoting surface K+ storage [5, 38], which also makes a great contribution to the high rate capability and long cycle life.
Figure 6. Schematic of the mixed K+ storage mechanism of the Sb-G-C nanofibers.
Download figure:
Standard image High-resolution imageActually, noteworthy is that choosing a suitable graphene concentration is of great importance for improving the electrochemical performances. In view of this, Sb-G-C composites with 0.05 and 0.2 ml GO/DMF adding into the precursor solutions were independently fabricated, to explore the effect of graphene concentration on collaborative K+ storage. On condition that the weight loss of GO is about 30% during the reduction process to graphene, while 5.1% weight loss for the PAN nanofibers is generated ascribed to the heat treatment till 700 °C [66, 67], the weight percentage of graphene in the Sb-G-C anodes can be roughly speculated as 0.0027 wt%, 0.0054 wt% and 0.0107 wt% when prepared with 0.05, 0.1, and 0.2 ml GO/DMF, respectively. The cycling abilities of cells with the four samples as binder-free anodes at 100 mA g−1 were compared (presented in figure S7). As summarized in figure 7(a), the Sb-G-C nanofibers with 0.05, 0.1 and 0.2 ml GO/DMF respectively exhibit the reversible capacities of 168, 204.95 and 182.61 mAh g−1 after 100 cycles, while the Sb-C counterpart only retains 121.54 mAh g−1, highlighting the boosted cycling stability by graphene modification. Specifically, 0.1 ml GO/DMF incorporation corresponds to the best storage condition. A similar result has also been observed in our previous study [31]. The superiority is associated with the moderate graphene content, which promotes the graphitization of amorphous carbon. This benefits the electrical properties of the carbon composites. Nevertheless, in contrast to the non-graphitic carbon with isotropic and disordered structure, associated with a relatively large number of active sites to accommodate the encapsulants and the insertion/extraction reaction of K+ [68], the graphitic carbon presents a poorer compatibility with the encapsulated metal nanoparticles, which have an increased tendency to aggregate or precipitate, and bring declined storage performances. Hence, the graphene content is a critical factor for regulating the electrochemical properties of the final product. Simultaneously, the rate capability tests in figure 7(b) provide further proof that 0.1 ml GO/DMF is the best selection.
Figure 7. Electrochemical performances comparison of (a) discharge capacities after 100 cycles, and (b) rate capabilities for the Sb-G-C composites with different graphene concentrations.
Download figure:
Standard image High-resolution image4. Conclusions
In summary, composites of Sb nanoparticles encapsulated in carbon nanofibers and modified by graphene via a facile electrospinning method were prepared. The synthesized Sb-G-C composites functioned as binder-free anodes for KIBs, providing an extraordinary cyclic stability (204.95 mAh g−1 over 100 cycles at 100 mA g−1) as well as the excellent rate capacity (120.83 mAh g−1 at 1 A g−1). The enhanced electrochemical performances of the Sb-G-C are bound up with the synergy effects from a rational design: (1) the fibrous coated carbon and the incorporated graphene exert important effect on restraining the violent structural fluctuation during cycling; (2) the fast ion transportation afforded by graphene renders high rate capability; (3) the dominant pseudocapacitive contribution largely arising from the porous nanostructure is favorable to improving the reversible capacity, the fast kinetics and the long-term cyclability. This work enriches the possibilities for the flexible Sb-G-C nanofibers as a self-supporting electrode material with high-performance potassium storage. We believe this work can provide insights to guide the development of the analogues for future application in rechargeable KIBs.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 51404103, 51574117 and 61376073), and Natural Science Foundation of Hunan Province (2017JJ5044).